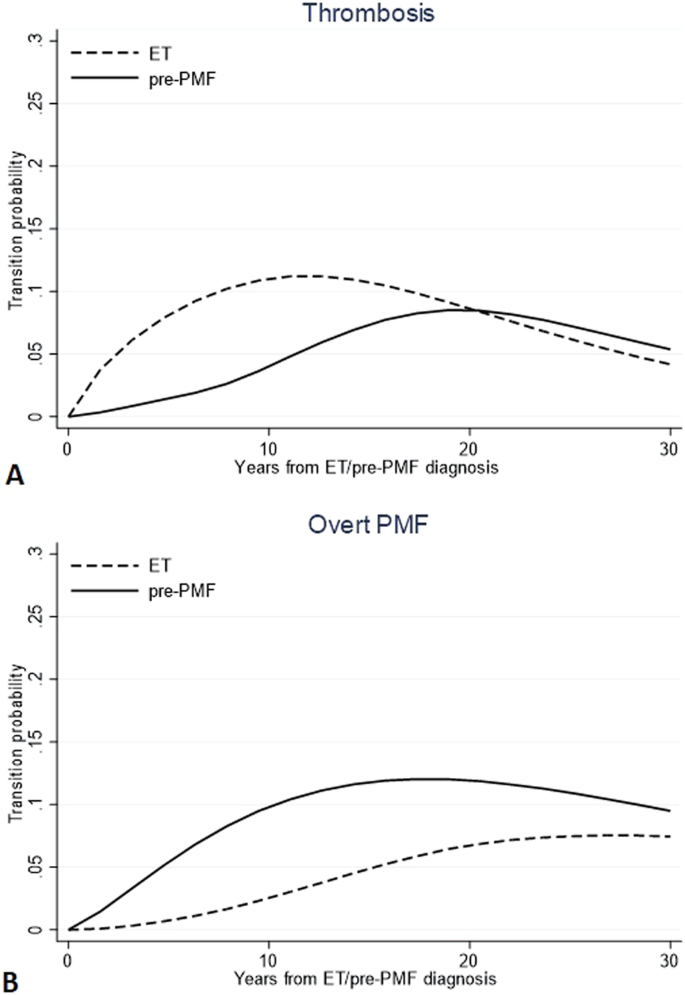
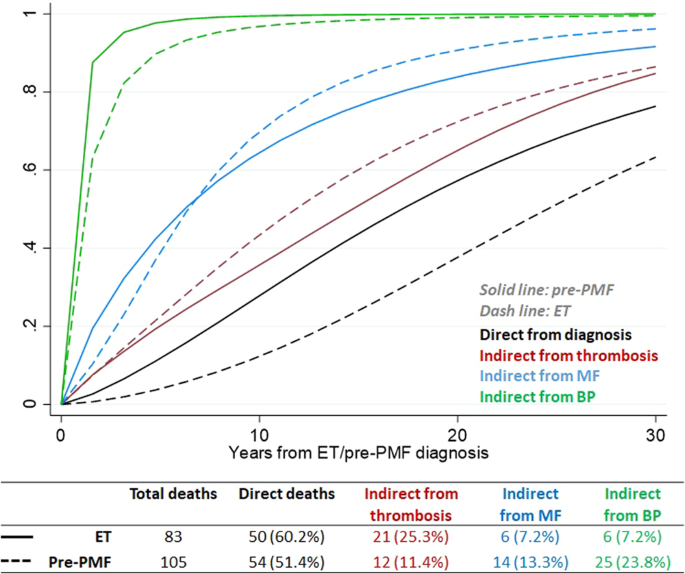
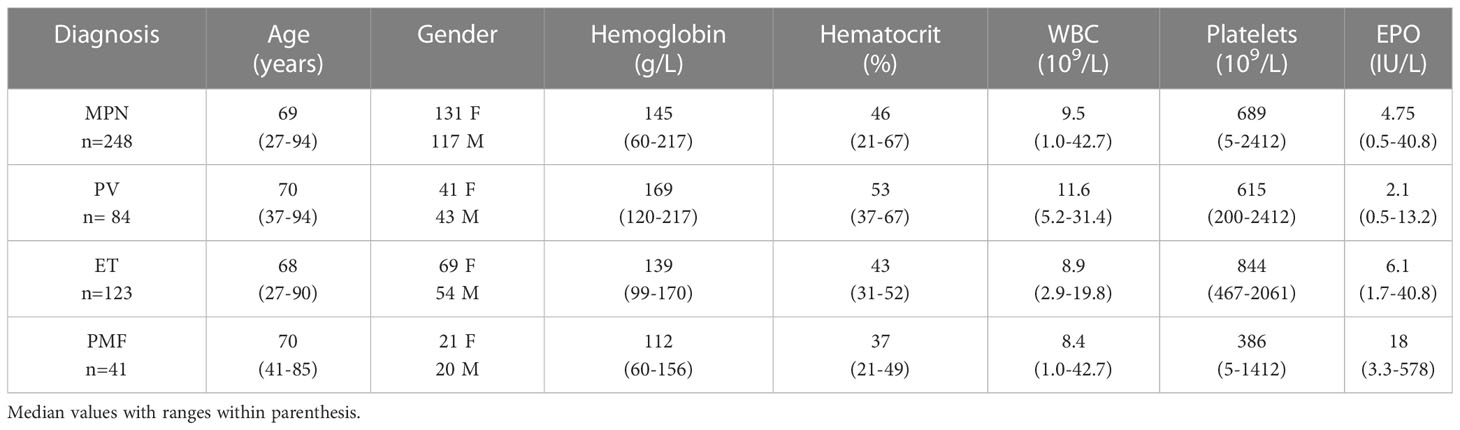
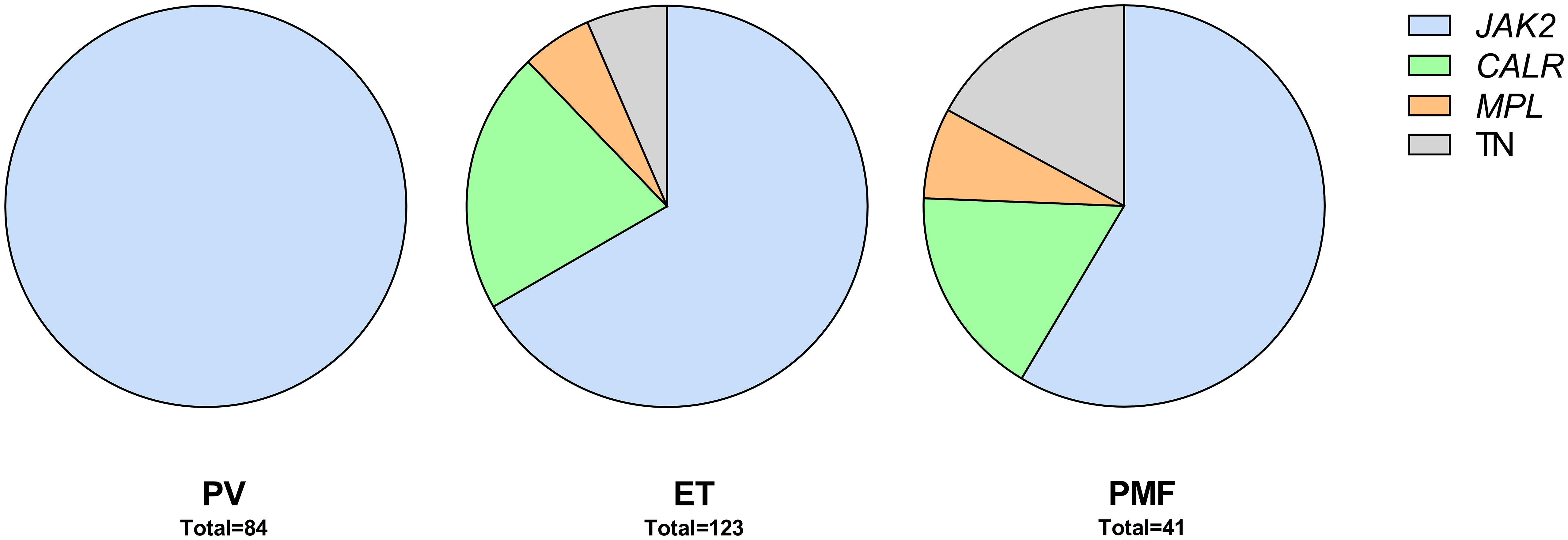
Research By: Mohammad Azam, PhD
Post Date: August 21, 2023
Cincinnati Children’s experts show, in mice, that targeting DUSP1 eradicates JAK2 mutated MPN
Myeloproliferative neoplasms (MPNs) are malignant bone marrow diseases that cause dangerous overproduction of red blood cells, white blood cells, and/or platelets. These conditions mostly strike adults around age 60 but can occur at any age. Some of these patients ultimately develop acute myeloid leukemia (AML).
Based on successes achieved in treating chronic myeloid leukemia (CML) with a class of drugs called ABL tyrosine kinase inhibitors (TKI), cancer researchers had high hopes that a similar class of drugs called JAK2 inhibitors would be a breakthrough for treating MPNs. However, clinical studies have found that JAK2 inhibitors are ineffective.
Now, a study recently published in the journal Leukemia reports achieving curative response in mice when they selectively knock-out a negative regulator of MAPK signaling: DUSP1. This highly complex study took a team of scientists at three institutions seven years to complete. The work was led by senior author Mohammad Azam, PhD, Divisions of Cancer Pathology and Experimental Hematology and Cancer Biology.
“This study, for the first time, provides mechanistic understanding why JAK2 inhibitors are ineffective in vivo and how JAK2V617F signaling suppresses P53 function required for MPN transformation and progression,” Azam says. “Selective targeting of DUSP1 opens up a completely novel therapeutic approach and a potentially curative treatment outcome in MPNs.”
OVERCOMING DEAD ENDS
Inspired by the clinical efficacy of TKI therapy for treating CML, a race began to identify similar molecular drivers in MPN that could be targeted for intervention.
Scientists initially found mutations of interest within three genes JAK2, MPL, and CALR. Further study of mouse genetic models revealed that all the mutations produced a common outcome: elevated and persistent JAK-STAT and MAPK signaling. This provided a strong rationale for developing small molecule inhibitors to target JAK2 kinase activity.
Numerous JAK inhibitors have been assessed in MPN and myelofibrosis (MF), another rare, chronic blood cancer. So far, three JAK2 inhibitors are approved by the FDA to treat MPN and MF while almost a dozen JAK inhibitors are currently undergoing pre-clinical and clinical assessment for potentially treating conditions such as arthritis, psoriasis, inflammation, graft-versus-host disease (GVHD), and autoimmune disorders.
However–unlike the success of TKI therapy in CML–JAK2 inhibitors do not induce remission. Instead, they simply slow cell division. Similarly, inhibitors targeting the MAPK pathway by blocking MEK1/2 or ERK1/2 either alone or in combination with JAK2 inhibitors failed to induce remission.
“Even the most potent kinase inhibitors failed to kill MPN cells in vivo,” Azam says.
It became clear that other mechanisms must be involved in preventing the effectiveness of JAK2 inhibitors. In prior studies, Azam’s lab had explored another mouse model of MPN that involved a different cancer cell growth factor called BCR-ABL kinase. That work revealed that growth-factor signaling in the context of oncogenic signaling induces the expression of c-FOS and DUSP1 that causes resistance to TKI treatment.
Inflammatory cytokine signaling is one of the cardinal features of MPN, with about 60 different cytokines induced in the context of these conditions. Azam reasoned that inflammatory cytokine signaling drives TKI persistence in JAK2 targeted MPNs.
ZEROING IN ON DUSP1
In addition to Azam, the research team on this project included first author Meenu Kesarwani, PhD, Division of Pathology; H. Leighton Grimes, PhD, director of the Cancer Pathology Program; and six other members of the pathology division at Cincinnati Children’s. Experts from the Medical College of Wisconsin and the Memorial Sloan-Kettering Cancer Center also contributed.
The team worked for seven years to conduct numerous experiments to tease apart the reasons for the persistent cellular resistance to JAK2 inhibitors in MPN. The co-authors conducted an extensive set of genetic analyses that revealed deregulation of 19 genes in TKI resistant cells. Ultimately, the team focused on DUSP1 because this gene appears to dampen the MAPK signaling that suppresses the P53 apoptotic pathway.
NOVEL APPROACH FOR MPN THERAPY
Importantly, their work revealed that mice lacking DUSP1 exhibit normal growth and reproduction, thus supporting the notion that a treatment targeting this gene’s function would have minimal side effects. When mice without the DUSP1 gene were further tested, the team gained crucial insight into the cell signaling mechanisms that help MPNs resist JAK2 inhibitors.
“In essence, inflammatory cytokine and JAK2V617F signaling converge to induce the expression of DUSP1, which prevents the function of P53.” (See figure)
P53 is often referred to as the “guardian of the genome” due to its role in regulating diverse external or internal stresses, such as DNA damage, activation of oncogenes, nutrient deprivation, and hypoxia. Importantly, it plays a critical role in deciding the cell fate, cell death or division arrest for DNA repair. Consequently, it plays a significant role in treatment outcomes to chemotherapy as most resistant patients harbor P53 inactivating mutations.
NEXT STEPS
While the genes and signaling pathways involved in the mouse research also appear to exist in humans, much more research is needed to determine whether a selective eradication of DUSP1 can be achieved to cure JAK2-induced MPN, Azam says.
Meanwhile, co-authors say the new discoveries about how to control growth factor signaling in MPN cells may also lead to improved treatments for other forms of cancer.