Patients with myelofibrosis have limited effective treatment options and a poor prognosis. However, the first-in-class telomerase inhibitor imetelstat is poised to expand the treatment armamentarium should it prove safe and effective in the newly initiated phase 3 IMpactMF trial (NCT04576156).1,2
Three FDA-approved treatment options are available for myelofibrosis: ruxolitinib (Jakafi), pacritinib (Vonjo), and fedratinib (Inrebic).1,3 However, a significant portion of patients discontinue treatment with 1 or more of these JAK inhibitors and the median overall survival (OS) with these agents ranges from 11 to 16 months, underscoring the need for more effective alternatives.1,3
“Not a lot of the therapies that we give are clearly anticlonal or antistem cell; [imetelstat] is a stem cell–directed therapy,” John O. Mascarenhas, MD, director of the Center of Excellence for Blood Cancers and Myeloid Disorders and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York, New York, said in an interview with OncologyLive®. “Telomerase is a great target because it is overexpressed constitutively in the myelofibrosis stem cells and only transiently in the normal stem cells. It adds these telomere repeats to chromosomes; every time cells divide you lose a certain amount of these caps, then the cells go into a quiescent state or undergo apoptosis. This is a way of leveling the playing field so that you no longer allow this sort of mechanism of immortality to the malignant stem cells.”
Imetelstat Shows Early-Stage Activity
Imetelstat was evaluated in patients with relapsed or refractory intermediate-2 or high-risk myelofibrosis at 2 dose levels in the phase 2 IMbark trial (NCT02426086). IMbark enrolled patients previously treated with a JAK inhibitor who had disease progression and an ECOG performance status of 2 or less, among other enrollment criteria. Those who were intolerant to a JAK inhibitor were not enrolled unless they satisfied the relapsed- or refractory-related criteria.3
Patients were randomly assigned to receive the active dose of imetelstat, which was determined in a pilot study to be 9.4 mg/kg (n = 59) or the minimally active dose with telomerase target engagement of 4.7 mg/kg (n = 48). The agent was given via a 2-hour intravenous infusion once every 3 weeks until disease progression, unacceptable toxicity, consent withdrawal, or lack of response.
The coprimary end points of the trial were 24-week spleen and symptom response rates. Secondary end points included OS, safety, and clinical improvement. Molecular response and changes in telomerase activity and human telomerase reverse transcriptase levels served as exploratory end points.
Findings showed that the 24-week spleen response rate was 10.2% and the 24-week symptom response rate was 32.2% among patients who received the 9.4 mg/kg dose. In the 4.7-mg/kg cohort, the 24-week rates were 0% and 6.3%, respectively. At a median follow-up of 27.4 months, the median OS was 29.9 months (95% CI, 22.8-not estimable [NE]) and 19.9 months (95% CI, 17.1-NE) in the 9.4-mg/kg and 4.- mg/kg cohorts, respectively. The 12-month survival rate was 84.0% (95% CI, 71.6%-91.4%) vs 78.6% (95% CI, 63.9%-87.9%), respectively. The 24-month survival rate was 57.5% (95% CI, 43.2%-69.5%) vs 41.8% (95% CI, 27.1%-55.8%), respectively.3
Patients treated at either dose level experienced benefit with imetelstat if they displayed at least 1 grade or higher improvement in bone marrow fibrosis. Patients who showed improvement (n = 19) experienced a median OS of 31.6 months (95% CI, 23.6-NE) compared with 24.6 months (95% CI, 18.4-NE) among 38 patients who showed no improvement (HR, 0.54; 95% CI, 0.23-1.29).
The study authors noted that imetelstat displayed an acceptable safety profile for this patient population. The most common treatment-emergent adverse events (TEAEs) of any grade in the lowerdose arm included diarrhea (38%), anemia (31%), nausea (31%), and peripheral edema (27%). In the 9.4-mg/kg arm, common any-grade TEAEs included thrombocytopenia (49%), anemia (44%), neutropenia (36%), and nausea (34%).3
TEAEs of grade 3 or worse severity in the 4.7-mg/kg arm included anemia (31%), thrombocytopenia (23%), and dyspnea (13%); in the higher-dose arm, grade 3 or higher TEAEs were thrombocytopenia (41%) anemia (39%), and neutropenia (32%).3
“The myelosuppression that’s there is manageable,” Mascarenhas said. “Other than that, [imetelstat] doesn’t have a significant signal of toxicity from nonhematologic aspects. We didn’t see real concerns from the pattern of toxicity, which was originally a concern for the drug. Sometimes you get some low-grade gastrointestinal toxicity, but I have to say it’s very rarely a reason for concern or discontinuation. From a nonhematologic standpoint, it seems well tolerated.”
The study authors concluded that imetelstat displayed clinical benefits with potential disease-modifying activity at the 9.4-mg/kg dose level and that the novel telomerase mechanism of action offers a new treatment option for patients with myelofibrosis that may alter the course of their disease.3
Notably, imetelstat also has shown activity in myelodysplastic syndrome (MDS), a cousin of myelofibrosis, according to Mascarenhas. Patients with low- or intermediate-1–risk MDS achieved significant and durable transfusion independence when treated with imetelstat compared with placebo, according to topline findings from the phase 3 IMerge trial (NCT02598661).4
Data from the primary analysis of IMerge showed that patients treated with imetelstat (n = 118) achieved a transfusion independence rate of 39.8% (95% CI, 30.9%-49.3%) at 8 weeks compared with 15.0% (95% CI, 7.1%-26.6%) among patients (n = 60) who received placebo (P < .001). Moreover, the 24-week transfusion independence rates were 28.0% (95% CI, 20.1%-37.0%) and 3.3% (95% CI, 0.4%-11.5%), respectively (P < .001).
Additionally, the median transfusion- independence duration reported with imetelstat was approximately 1 year vs approximately 13 weeks with placebo via Kaplan-Meier estimates, indicating statistically significant durable transfusion independence for 8-week transfusion-independent responders (HR, 0.23). In the 24-week transfusion-independent responders who received imetelstat, the median transfusion independence duration was approximately 1.5 years.
These findings met the trial’s primary end point of percentage of patients without any red blood cell (RBC) transfusions during any consecutive 8-week period; the key secondary end point of percentage of patients without RBC transfusions in a 24-week period also was met.
“Durable transfusion independence [was observed] across the spectrum of patients,” Mascarenhas said. “That opens up the potential pathway for approval in MDS with a goal of addressing anemia. It’s interesting because in myelofibrosis we’re really [administering] it as a therapy to try to prolong survival in this very advanced sick patient population; MDS is sort of the other way around, [where it’s given] to patients and the goal is [management of] anemia, but even in that study they saw molecular responses.”
IMpactMF Looks to Solidify Imetelstat’s Place in Myelofibrosis
Following the positive findings from IMbark, investigators initiated the phase 3 randomized, open label, multicenter IMpactMF trial comparing the efficacy and safety of imetelstat with that of best available therapy (Figure1,5).
Figure. IMpactMF Phase 3 Trial Design1,5
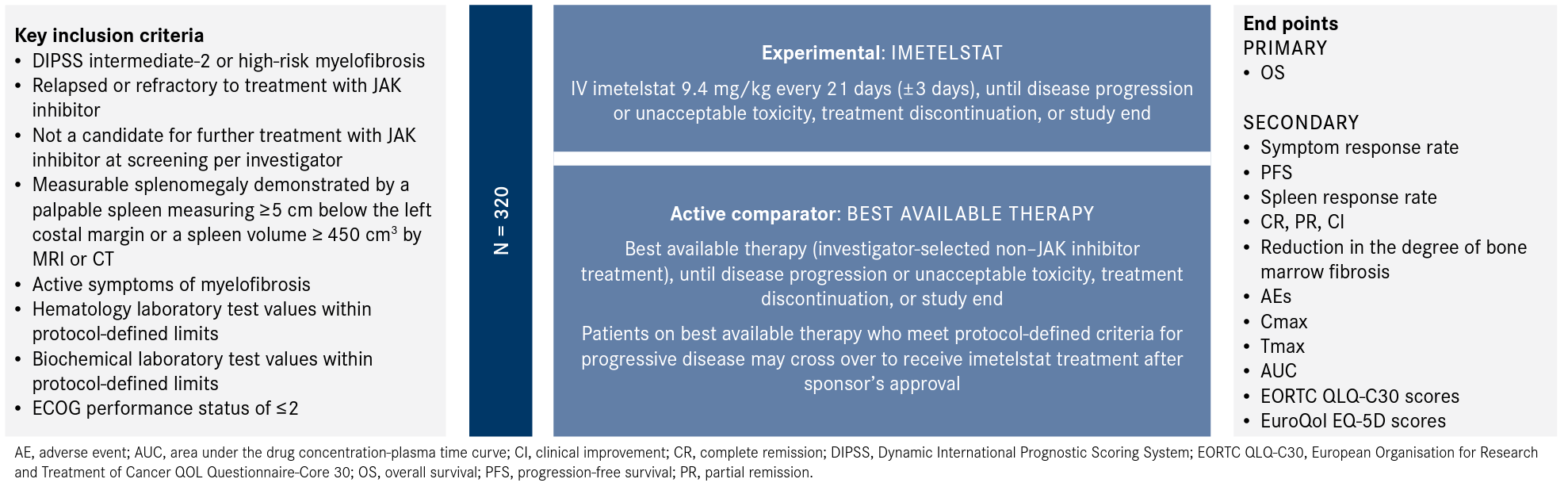
The study aims to enroll a total of approximately 320 adult patients with intermediate-2 or high-risk myelofibrosis that is relapsed or refractory to treatment with a JAK inhibitor. Patients are eligible for enrollment if they have an ECOG performance status of 2 or less, have active myelofibrosis symptoms with a symptom score of at least 5 points according to the Myelofibrosis Symptom Assessment Form version 4.0, and have hematology and biochemical test values within the protocol defined limits. Patients with a peripheral blood blast count or a bone marrow blast count of 10% or more, with prior treatment with imetelstat, or who have undergone major surgery within 28 days prior to randomization will be excluded from enrollment.1,5
Eligible patients will be randomly assigned 2:1 to receive either imetelstat or best available therapy, which will consist of an investigator-selected nonJAK inhibitor treatment. Imetelstat at 9.4 mg/kg or best available therapy will be given every 3 weeks until disease progression or unacceptable toxicity, treatment discontinuation, or study end. Patients in the control arm will have the option to cross over to receive imetelstat if they meet the protocol-defined criteria for progressive disease.
The primary end point is OS, and secondary end points include symptom response rate, progression-free survival, and spleen response rate. The trial is recruiting patients and is estimated to be completed in August 2025.
“This is [enrolling] patients who have really bad disease—it’s an unmet need; they don’t have excellent choices at that point [when they] are refractory to ruxolitinib,” Mascarenhas said. “I’m not aware of [other] studies in myelofibrosis where survival is an end point; usually it’s spleen and symptom benefit.
“We are in desperate need of therapies that can prolong survival in this patient population. For some patients, it might be a bridge to transplant, which is definitive and potentially curative. For other patients, it may simply be to improve survival, maybe improve their disease process default, [or] their malignant stem cell population. Maybe [other patients would] move on to other combination therapies—there are a lot of combination therapies moving forward in this space.”
References
- Mascarenhas J, Harrison C, Kiladjian JJ, et al. MYF3001: a randomized open label, phase 3 study to evaluate imetelstat versus best available therapy in patients with intermediate-2 or high-risk myelofibrosis relapsed/refractory to janus kinase inhibitor. Blood. 2022;140(suppl 1):6826-6829. doi:10.1182/blood-2022-160364
- Imetelstat. Geron. 2023. Accessed February 28, 2023. https://www.geron.com/research-and-development/imetelstat/
- Mascarenhas J, Komrokji RS, Palandri F, et al. Randomized, single-blind, multicenter phase II study of two doses of imetelstat in relapsed or refractory myelofibrosis. J Clin Oncol. 2021;39(26):28812892. doi:10.1200/JCO.20.02864
- Geron announces positive top-line results from IMerge phase 3 trial of imetelstat in lower risk MDS. News release. Geron. January 4, 2023. Accessed March 1, 2023. https://ir.geron.com/investors/press-releases/press-release-details
- A study comparing imetelstat versus best available therapy for the treatment of intermediate-2 or high-risk myelofibrosis (MF) who have not responded to janus kinase (JAK)-inhibitor treatment. ClinicalTrials.gov. Updated January 13, 2023. Accessed March 1, 2023. https://clinicaltrials.gov/ct2/show/NCT04576156
